Explain the shape of SF6 according to VSEPR theory
Answers
Answered by
20
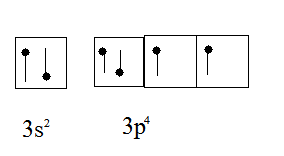
S has electron configuration as 
{image below 1st}
When 6 F's come the electron jumps from 3s & 3p to 3d
{image below 2nd}
The flourine gets placed there forming hybridization...
hybridization...
Thus the shape is octahedral...
{image below 1st}
When 6 F's come the electron jumps from 3s & 3p to 3d
{image below 2nd}
The flourine gets placed there forming
Thus the shape is octahedral...
Attachments:


Answered by
8
The Sf6 the shape of sf6 is octahedral it was calculated by number of lone pair and Bond pair
Similar questions